Negative Pressure Wound Therapy
A multi-centre phase III randomised controlled trial, comparing NPWT against current standard practice to reduce SSI’s following emergency laparotomy.

The UK trial is led by the Trainee Research Collaboratives in the West Midlands and North West Regions of England. Sunrrise Australia has full support from NIHR RfPB board, the UK trial leaders and the Clinical Trials Network Australia and New Zealand (CTANZ)
This study tests whether a new type of active wound dressing can reduce rates of wound infection (surgical site infection, ‘SSI’) after emergency surgery on the abdomen. These dressings are Single Use Negative Pressure Dressings (SUNPD). They combine a sealed pad and suction to remove fluid from in and around the wound.
Emergency abdominal operations are performed for a variety of life-threatening reasons, including bowel blockages, perforations, traumatic injuries and infections. At least 1 in 4 patients will be affected by an SSI after these procedures. These SSIs are painful, take longer to heal, extend hospital stays, incur significant extra costs, and can lead to ongoing wound care in the community. In an already unwell patient, development of an SSI can contribute to other major complications, including death.
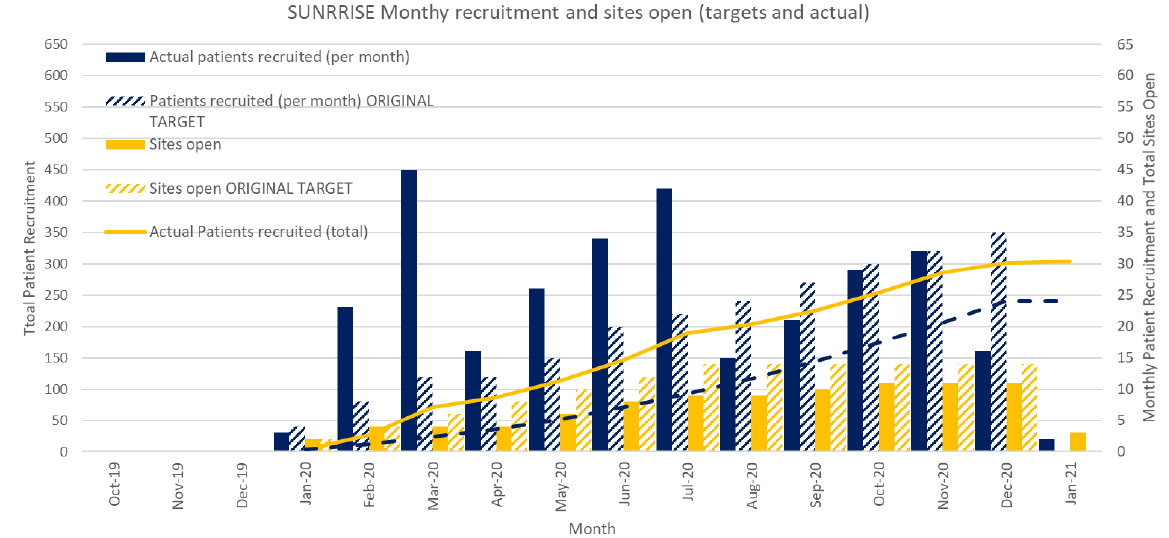
The trial is currently recruiting patients at 5 sites in the UK. It is expected that a further 25 UK sites will be opened by December 2019. With support from this grant, Australian sites will start recruiting in October 2019
The Australian study increases the power from 80% to 90%, reinforces the links in Surgical Research that have developed between the Research Collaboratives in the UK and Australia, and enhances surgical clinical research capacity in Australia by training a new generation of surgical trainees in trial methodology and conduct.
Aim:
A multi-centre phase III randomised controlled trial, comparing NPWT against current standard practice to reduce SSI’s following emergency laparotomy.
Outcomes:
Primary outcome: occurrence of an SSI within 30 days following surgery.
Rationale:
High risk population with multiple co-morbidities such as diabetes, obesity and cardiac disease which increase their risk of developing an SSI.
Future RCT
A multi-arm, multi-stage RCT focussing on a high risk population – vascular patients undergoing any lower limb operations.